Humoral immune response against SARS-CoV-2 variants
SARS-CoV-2 variants could induce immune escape by mutations on the receptor-binding domain (RBD) and N-terminal domain (NTD). Here we report the humoral immune response to circulating SARS-CoV-2 variants, such as 501Y.V2 (B.1.351), of the plasma and neutralizing antibodies (NAbs) elicited by CoronaVac (inactivated vaccine), ZF2001 (RBD-subunit vaccine) and natural infection.
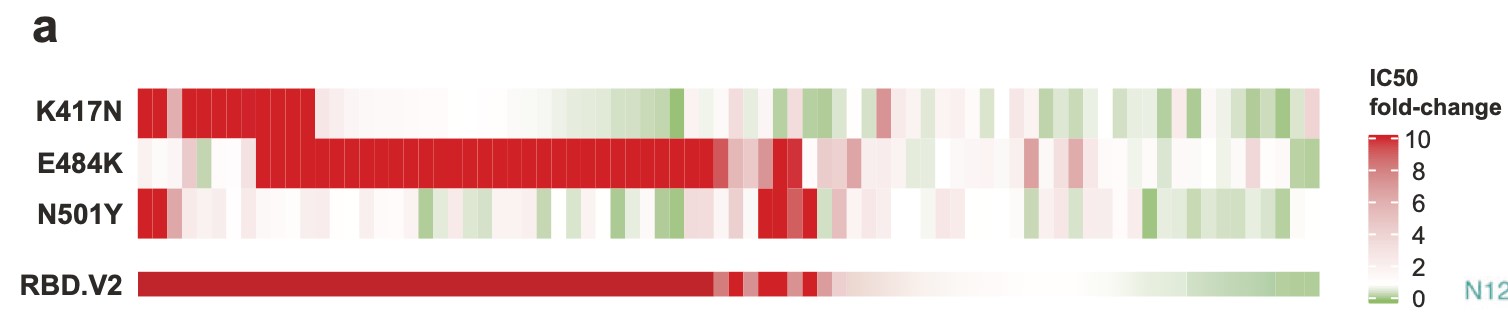
Among 86 potent NAbs identified by high-throughput single-cell VDJ sequencing of peripheral blood mononuclear cells from vaccinees and convalescents, near half anti-RBD NAbs showed major neutralization reductions against the K417N/E484K/N501Y mutation combination, with E484K being the dominant cause (Fig.1).
Fig. 1. Responses of anti-RBD and anti-NTD SARS-CoV-2 NAbs to 501Y.V2. IC50 fold-changes of 80 potent anti-RBD NAbs against pseudovirus carrying 501Y.V2 RBD mutations.
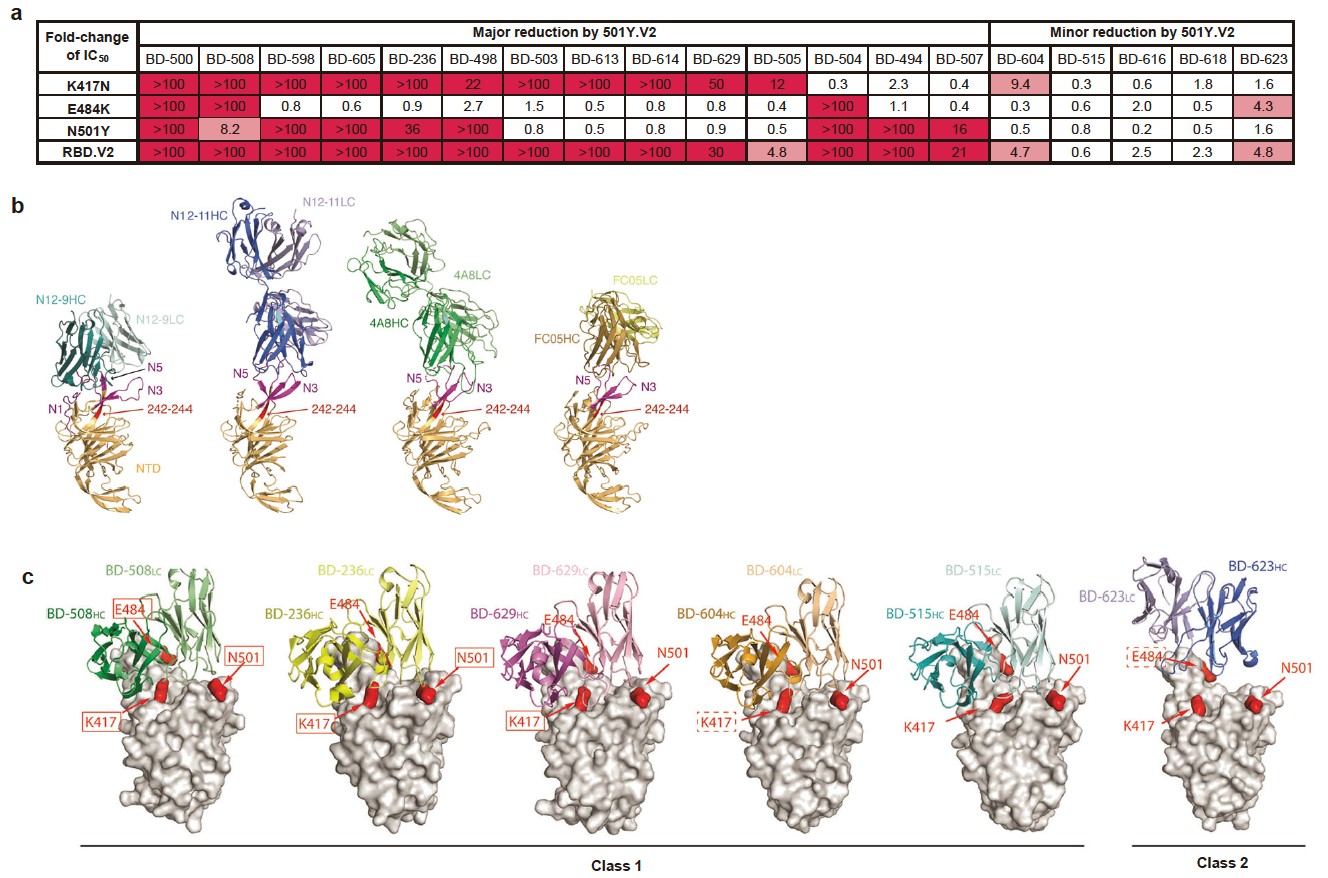
VH3-53/VH3-66 recurrent antibodies respond differently to RBD variants, and K417N compromises the majority of neutralizing activity through reduced polar contacts with complementarity determining regions (Fig.2a). In contrast, the 242–244 deletion (242–244Δ) would abolish most neutralization activity of anti-NTD NAbs by interrupting the conformation of NTD antigenic supersite, indicating a much less diversity of anti-NTD NAbs than anti-RBD NAbs (Fig.2b). To assess the origin of diversity of anti-RBD Nabs, we performed structural analyses (Fig. 2c). Due to difference in their binding epitopes, these NAbs exhibit different degrees of sensitivity to 501Y.V2. Some of the NAbs are extremely vulnerable to the mutations of Lys417, Glu484, and Asn501, whereas others are more tolerant.
Fig. 2. a. IC50 fold-changes of VH3-53/VH3-66 NAbs against pseudovirus carrying 501Y.V2 RBD mutations. Red indicates major fold-change larger than 10-fold. Pink indicates minor fold-change between 3- and 10-fold. b. Structure analyses of the NTD NAbs. The N1, N3, and N5 loops of NTD are highlighted in magenta. Residues 242–244 are highlighted in red. c. Structure analyses of the interaction between VH3-53/VH3-66 NAbs and RBD. RBD is shown as a surface view, whereas the NAbs are shown as ribbons. Red solid rectangles indicate that the mutation would result in a major decrease in antibody binding. Red dashed rectangles indicate that the mutation would result in a minor decrease in antibody binding.
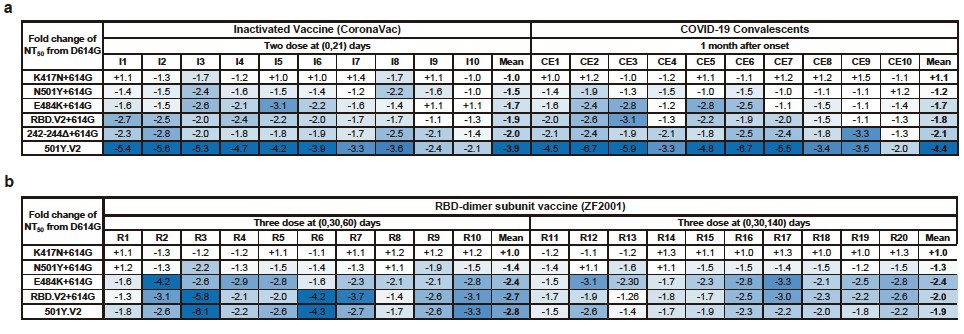
Plasma of convalescents and CoronaVac vaccinees displayed comparable neutralization reductions against pseudo- and authentic 501Y.V2 variants, mainly caused by E484K/N501Y and 242–244Δ, with the effects being additive (Fig.3a). Importantly, RBD-subunit vaccinees exhibit markedly higher tolerance to 501Y.V2 than convalescents, since the elicited anti-RBD NAbs display a high diversity and are unaffected by NTD mutations (Fig.3b). Moreover, an extended gap between the third and second doses of ZF2001 leads to better neutralizing activity and tolerance to 501Y.V2 than the standard three-dose administration (Fig.3b).
Fig. 3. Neutralization of 501Y.V2 by convalescent, CoronaVac vaccinee, and ZF2001 vaccinee plasma. a. Summary of the fold change of NT50 of convalescent and CoronaVac vaccinee plasma for the indicated mutants from D614G. Color gradient indicates fold change values ranging from +1 (white) to −6.7 (blue). b. Summary of the fold change of NT50 of ZF2001 vaccinee for the indicated mutants from D614G. Color gradient indicates fold change values ranging from +1 (white) to −6.1 (blue). All plotted values and horizontal bars in this figure indicate the geometric mean.
Together, these results suggest that the deployment of RBD-vaccines, through a third-dose boost, may be ideal for combating SARS-CoV-2 variants when necessary, especially for those carrying mutations that disrupt the NTD supersite.