Characterization of the enhanced infectivity and antibody evasion of Omicron BA.2.75
Recently emerged SARS-CoV-2 Omicron subvariant named BA.2.75 displayed a growth advantage over BA.5 in India. However, the underlying mechanisms for enhanced infectivity, especially compared to BA.5, remain unclear.
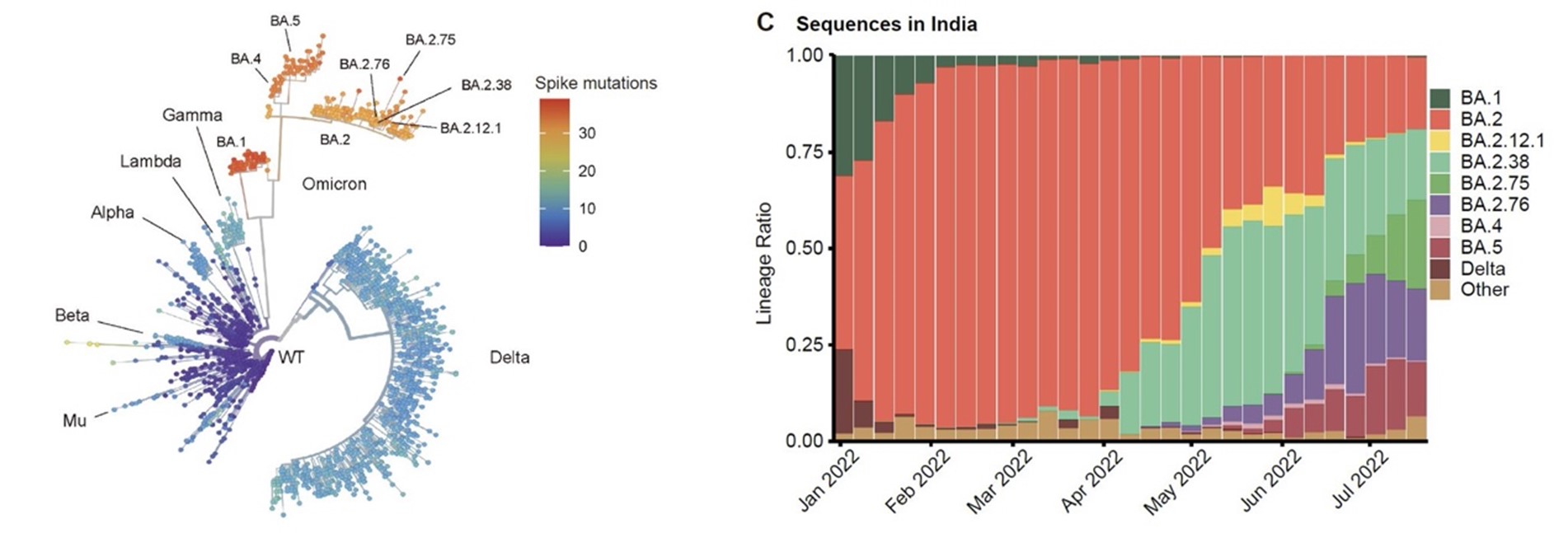
In this study, we show BA.2.75 exhibits substantially higher affinity for host receptor ACE2 than BA.5 and other variants. Structural analyses of BA.2.75 Spike shows its decreased thermostability and increased frequency of the receptor binding domain (RBD) in the “up” conformation under acidic conditions, suggesting enhanced low-pH-endosomal cell entry.
Relative to BA.4/BA.5, BA.2.75 exhibits reduced evasion of humoral immunity from BA.1/BA.2 breakthrough-infection convalescent plasma, but greater evasion of Delta breakthrough-infection convalescent plasma. BA.5 breakthrough infection plasma also exhibits weaker neutralization against BA.2.75 than BA.5, mainly due to BA.2.75’s distinct neutralizing antibody escape pattern. These results suggest BA.2.75 may prevail after BA.4/BA.5, and its increased receptor-binding capability could support further immune-evasive mutations.
One advantage of BA.2.75 is its extremely high hACE2-binding affinity, as determined by surface plasma resonance (SPR) assays. By solving the hACE2/Spike complex structure of BA.2.75, we showed that indeed R493Q reversion is the major contributor, independent of N460K.
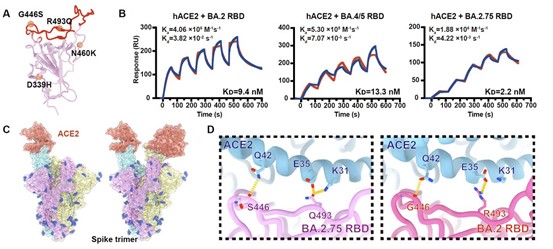
However, N460K established a new salt bridge with D420, together with the π-π interactions formed between D339H and F371, making BA.2.75 RBD display the most rigid and stable configuration compared to other Omicron variants.
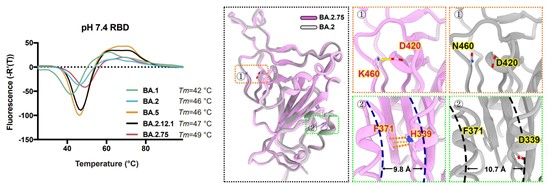
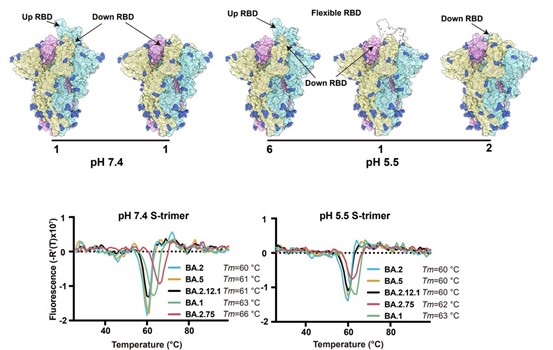
BA.2.75 prefusion spike at endosomal pH displayed substantially reduced stability and increased RBD “up” conformation than at serological pH, suggesting BA.2.75 may have evolved to further utilize the low-pH endosomal cell-entry pathway.
Most importantly, we found that BA.2.75 exhibits strong evasion in convalescent plasma from BA.5 breakthrough infection (~4-fold reduction in NT50 compared to the NT50 against BA.5). This would give BA.2.75 huge transmission advantage after the global BA.4⁄5 wave.
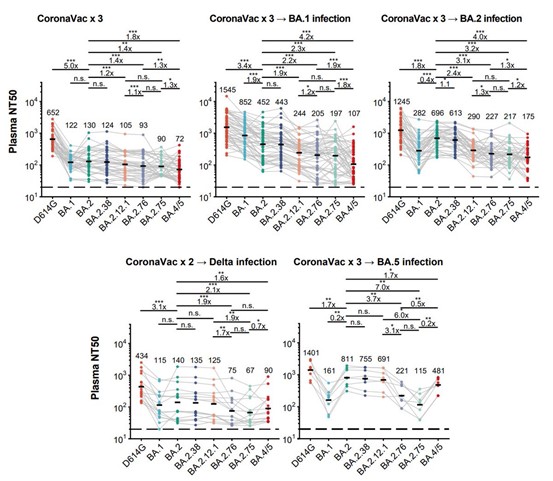
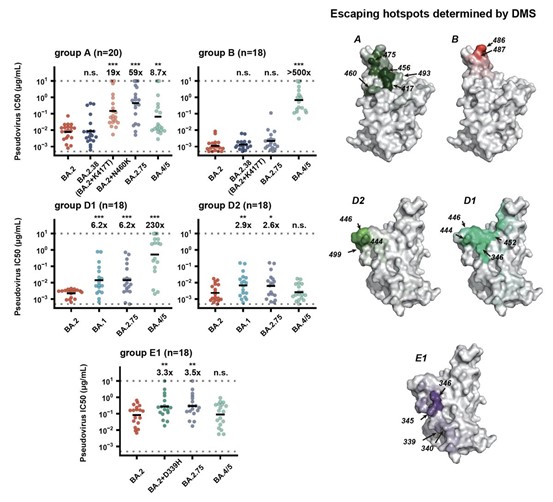
The striking evasion of BA.5 infection plasma by BA.2.75 is mainly due to the distinct RBD antigenicity and antibody escaping pattern between BA.4⁄5 (L452R, F486V) and BA.2.75 (G446S, N460K), as we have shown in previous publication.
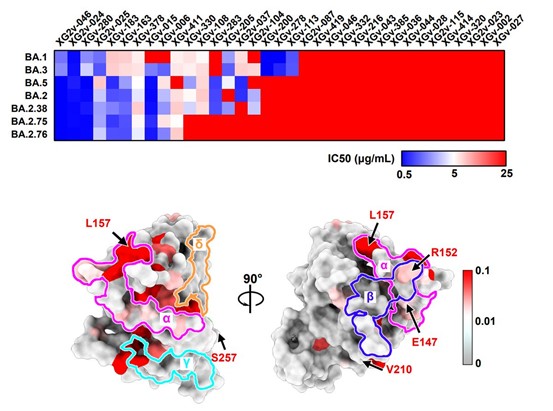
We found that BA.2.75 also display distinct NTD antigenicity and neutralizing antibody-escaping patterns compared to BA.2 and BA.5. The K147E, W152R and F157L mutations on BA.2.75 would escape BA.5-effective NTD neutralizing antibodies.
These results suggest BA.2.75 may prevail after the global BA.4/BA.5 wave, and its increased receptor-binding capability could allow further incorporation of immune-evasive mutations. In fact, BA.2.75 + R346T, BA.2.75 + L452 and BA.2.75 + L452 + K444M have already emerged.
One additional surprisingly finding is that, S309 displayed recovered RBD binding affinity and IC90 against BA.2.75 than BA.5. S309/BA.2.75 Spike complex structure reveals that D339H actually established more hydrophobic interactions, thus strengthening the binding.
This work was done in collaboration mainly with Prof. Xiangxi Wang’s group .
Reference: